What exactly is caffeine? How is that jolt of caffeine removed from coffee?
Caffeine is a mild stimulant found in more than 63 plants, including coffee beans, cocoa beans, kola nuts, and tea leaves. Caffeine is found most often in soda, chocolate, energy drinks, tea, various alertness pills, and, of course, coffee.
Anthropologists believe caffeine use may date back to the Stone Age.
 |
| Morning Coffee Francis Boucher 1739 |
Coffee has since been linked to religious ceremonies where people involved in the rituals could stay up and pray or worship most of the night. Coffee was introduced to the Europeans in 1573, and 250 years later in 1821, caffeine was first extracted from coffee.
Removing the caffeine “jolt”
Decaffeination uses green coffee beans. There are four methods of decaffeination, based on the substance used to extract the caffeine: (1) Water , (2) Ethyl Acetate, (3) Supercritical or Liquid CO2, and (4) Methylene Chloride.
These 4 methods share the following 4 stages:
· Stage 1: Swelling the beans water or steam to make the caffeine more available to be removed
· Stage 2: Removing the caffeine from the beans
· Stage 3: Steam stripping to remove all solvent residues from the beans (when applied) / regenerating adsorbents (when applied)
· Stage 4: Drying the decaffeinated coffee beans to return to their normal moisture content
Now, let’s look more closely at the 4 methods of decaffeinating green coffee beans.
Method 1 - Water Method: The green coffee beans are immersed in hot water anywhere from 10 minutes to 2 hours to leech out the caffeine. Much of the coffee’s aromatic character can be lost in this process, so workers saturate beans with the water-soluble components of the coffee. The caffeine is subsequently removed from the solution using activated carbon or other absorbents.
Method 2 - Ethyl - Acetate method: Ethyl - Acetate (EA) occurs in several natural products and contributes to the characteristic aroma of many fruit. EA is also found in varying concentrations in foodstuffs including green and roasted coffee. Because of the impracticality of gathering EA from natural sources, this process uses a synthetic version. The beans are first steamed for 30 minutes then rinsed repeatedly with ethyl acetate for about 10 hours. The solvent is then drained away and the beans steamed for an additional 10 hours to remove residual solvent.
Method 3- Supercritical Carbon Dioxide and Liquid Carbon Dioxide method: CO2 is a readily available substance of great purity, naturally available in the air we breathe. Under certain conditions CO2 can provide a selective caffeine extraction, leaving most of the other coffee bean components unaltered. Using CO2 in its supercritical state (between its liquid and gaseous state) requires very high pressure – up to 250 atmospheres, necessitating a large-scale production to be economically viable.
Method 4- Methylene Chloride (i.e. Dichloromethane-DCM) method: DCM is circulated through the water soaked beans. The resulting mixture of DCM and caffeine is drained out. This step is repeated several times, until the residual caffeine content is at or below the legal maximum level of 0.1%.
NEXT: Roasting the beans
http://myperfectcoffee.com
7WQY9ETSPNAW
· Stage 1: Swelling the beans water or steam to make the caffeine more available to be removed
· Stage 2: Removing the caffeine from the beans
· Stage 3: Steam stripping to remove all solvent residues from the beans (when applied) / regenerating adsorbents (when applied)
· Stage 4: Drying the decaffeinated coffee beans to return to their normal moisture content
Now, let’s look more closely at the 4 methods of decaffeinating green coffee beans.
 |
| Ethyl - Acetate (EA) |
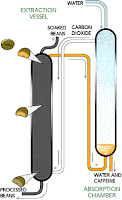 |
| CO2 Process |
Method 3- Supercritical Carbon Dioxide and Liquid Carbon Dioxide method: CO2 is a readily available substance of great purity, naturally available in the air we breathe. Under certain conditions CO2 can provide a selective caffeine extraction, leaving most of the other coffee bean components unaltered. Using CO2 in its supercritical state (between its liquid and gaseous state) requires very high pressure – up to 250 atmospheres, necessitating a large-scale production to be economically viable.
Method 4- Methylene Chloride (i.e. Dichloromethane-DCM) method: DCM is circulated through the water soaked beans. The resulting mixture of DCM and caffeine is drained out. This step is repeated several times, until the residual caffeine content is at or below the legal maximum level of 0.1%.
NEXT: Roasting the beans
http://myperfectcoffee.com
7WQY9ETSPNAW




No comments:
Post a Comment